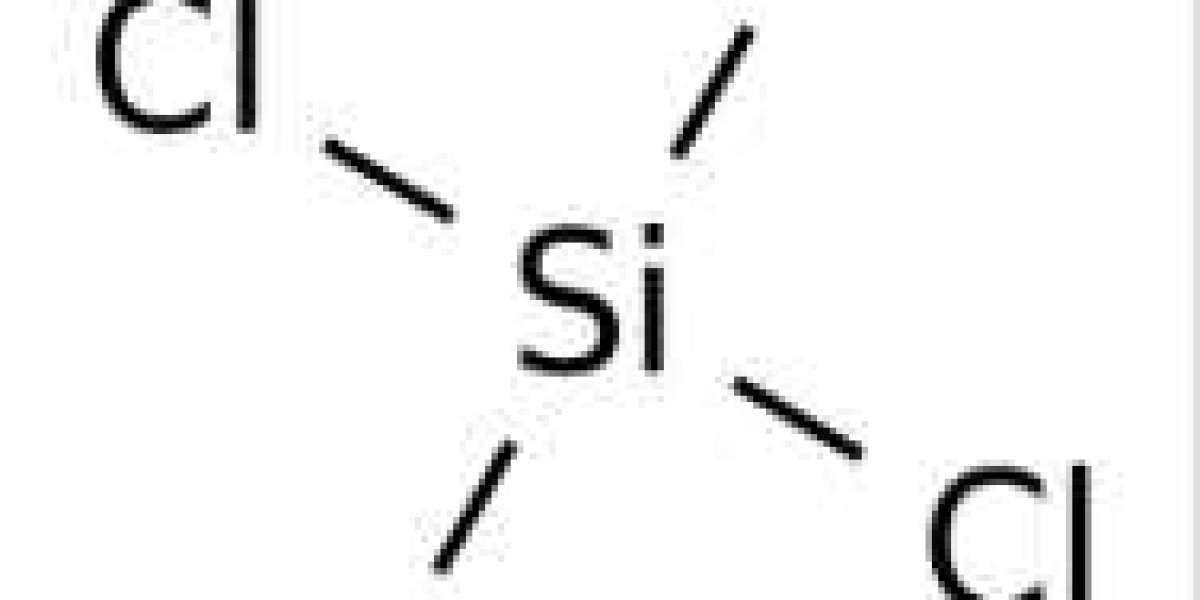Reaction with alcohols and ethanol to produce tetramethyl orthosilicate and tetraethyl orthosilicate:
SiCl4 + 4 ROH → Si(OR)4 + 4 HCl
At higher temperatures, silicon tetrachloride homologues can be prepared by the following reaction:
Si + 2 SiCl4 → Si3Cl8
In fact, the chlorination of silicon is accompanied by the formation of hexachlorodisilane Si2Cl6. A range of compounds containing up to six silicon atoms in the chain can be separated from a mixture using fractional distillation. [1]
Silicon tetrachloride is a classic electrophile in terms of its reactivity. [6] Formation of various organosilicon compounds after treatment with Grignard reagents and organolithium compounds:
4 RLi + SiCl4 → R4Si + 4 lithium chloride
Reduction with a hydride reagent affords the silane.
Silicon tetrachloride is used as an intermediate in the manufacture of polysilicon, an ultrapure form of silicon,[3] because its boiling point facilitates purification by repeated fractional distillation. It is reduced by hydrogen in the hydrogenation reactor to trichlorosilane (HSiCl3), which is used directly in the Siemens process or further reduced to silane (SiH4) and injected into the fluidized bed reactor. Silicon tetrachloride reappears in both processes as a by-product and is recycled in the hydrogenation reactor. Vapor phase epitaxy of hydrogen reduction of silicon tetrachloride at about 1250 °C has been accomplished: