Have you ever tried making a cake? If so, you’re probably aware that baking soda is one of the primary ingredients for a fluffy cake. The chemical name for baking soda is sodium bicarbonate melting point, and its molecular structure is NaHCO3.Usually available as a fine powder, baking soda has a white appearance. Nicolas Leblanc, a French scientist, created sodium carbonate, sometimes known as soda ash, in 1791. Fishermen used potassium and sodium bicarbonates to preserve fish in the 1800s. Later, in 1846, two American bakers named Austin Church and John Dwight produced baking soda for the first time at their factory using sodium carbonate and carbon dioxide.
Sodium Bicarbonate Production
The chemical is created on a large scale by reacting cold and concentrated brine (sodium chloride) solutions with CO2 and ammonia. The Solvay technique used to produce sodium carbonate looks like this (washing soda).
The chemical reaction: NaCl + H2O + CO2 + NH3 - NH4Cl + NaHCO3
One byproduct of this procedure is sodium hydrogen carbonate. It breaks down into sodium carbonate and carbon dioxide gas when heated to 373 K.
2NaHCO3 - Na2CO3 + H2O + CO2
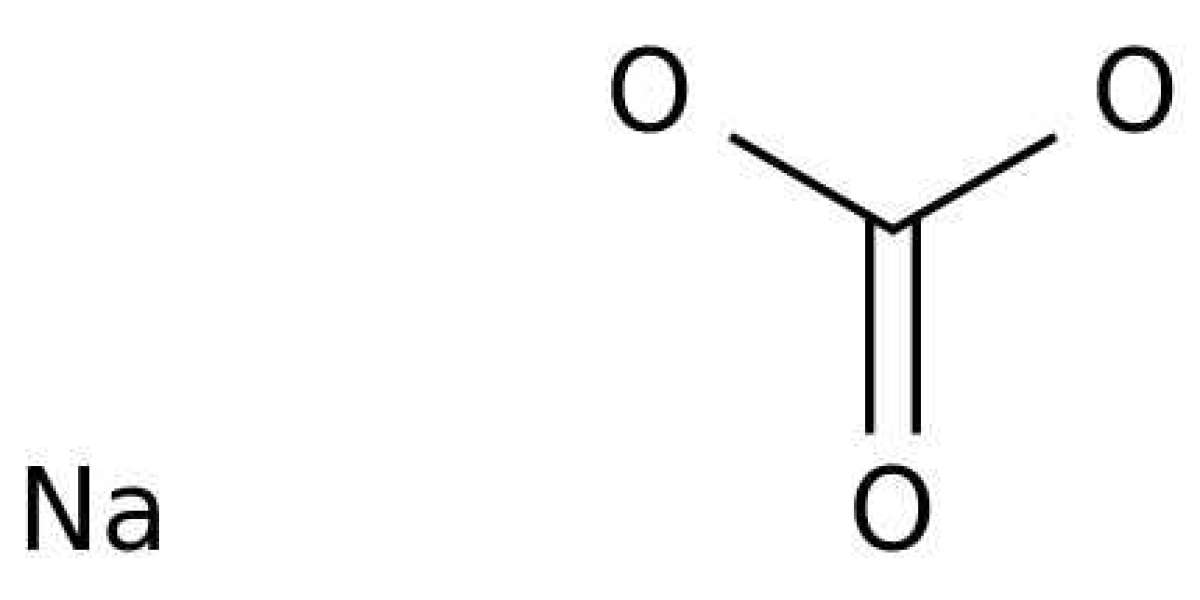
Sodium bicarbonate formula
The popular term for the substance sodium bicarbonate, also known as sodium hydrogen carbonate, is baking soda.NaHCO3 is Sodium bicarbonate formula. In water, the substance, which is a salt, separates into sodium (Na+) cation and carbonate (CO3–) anions. Typically marketed as a powder, baking soda is an alkaline, white, crystalline substance. It tastes a little salty.
The structure of Sodium Bicarbonate
1.NaHCO3 is the sodium bicarbonate formula.
2.A sodium cation (Na+) and a bicarbonate anion (HCO3-) are present.
3.It has a monoclinic lattice structure.
4.One sodium atom, one carbon atom, one hydrogen atom, and three oxygen atoms make up the compound.
Sodium bicarbonate uses
Baking soda is a multifunctional chemical. Because of its use and reasonable price, it has become a typical home item. Its numerous applications include:
1.At Home:
It is an excellent household chemical, primarily used as a cleaning agent, culinary ingredient, and disinfectant. Foods like chickpeas and yellow peas are frequently cooked faster by adding baking soda. Old carpets, rugs, cabinets, shoes, refrigerators, and vacuum cleaners can all benefit from its deodorizing properties. It may eliminate stubborn stains from the floor, clothing, and furniture. It serves as a water conditioner and softener and removes dirt from clothing when used in washing.
2.In battling fires and handling materials:
The white, combustible substance also puts out fires. It is the primary component of dry powder fire extinguishers and hand-held multipurpose extinguishers. Multipurpose extinguishers put out many kinds of flames in homes, workplaces, and automobiles. The makers and users of sanders have recently been interested in baking soda. For the preservation or removal of the top layer of many materials, including metal or wood, the loose, whitening sodium bicarbonate melting point is an excellent abrasive.
3.Athletic excellence:
Sodium bicarbonate used as an exercise aid has been the subject of research, although some have claimed that it might improve stamina levels, which could improve performance. By helping to buffer any acidic waste products that emerge during extended activity, the powder’s high pH level reduces the tiredness in those who are conducting high-intensity activities.
4.In the garden:
Sodium bicarbonate is used as a natural insecticide in the garden to eradicate dangerous insects and fungi that harm plants. You may add it to the soil to raise the pH level and make it more alkaline. Some plants favor soils that are alkaline. It is also beneficial for cleaning garden tools.
5.In the beauty sector:
The cosmetics sector also makes extensive use of baking soda. Toothpaste, deodorants, face packs, body and face washing foams, and gels include crystalline sodium bicarbonate melting point. In addition, it is a highly valued ingredient used in cosmetic powders, effervescent bath bombs, washes, and tablets used to clean braces or dental implants. Because sodium bicarbonate has a calming and skin-lightening effect, its uses: Before receiving treatments at spas or at home to cleanse hands and feet, The production of creams for the legs and hands, and Make hair masks to treat dandruff. New products for men, women, and kids based on sodium are in the making. Mouthwashes, whitening toothpaste, and rejuvenating lotions all include the inorganic substance.
6.In medicine:
It is used as an antacid to treat heartburn and indigestion. By lowering stomach acid, it acts quickly to offer momentary relief. Due to its alkaline composition, it reduces indigestion by neutralizing the excess hydrochloric acid in the stomach. Doctors use a 5% sodium bicarbonate infusion in medical crises such as severe renal failure, a heart attack, uncontrolled diabetes, and so. Cosmetics, personal hygiene items, and items for beauty care are all made using it. To lessen the negative effects of chemotherapy. Its antimicrobial characteristics make it ideal for cleansing the mouth and teeth.