Ammonium bicarbonate is an inorganic compound with the molecular formula (NH4)HCO3. The compound has many names, reflecting its long history. Chemically, it is the bicarbonate of ammonium ions. It is a colorless solid that readily degrades to carbon dioxide, water and ammonia.
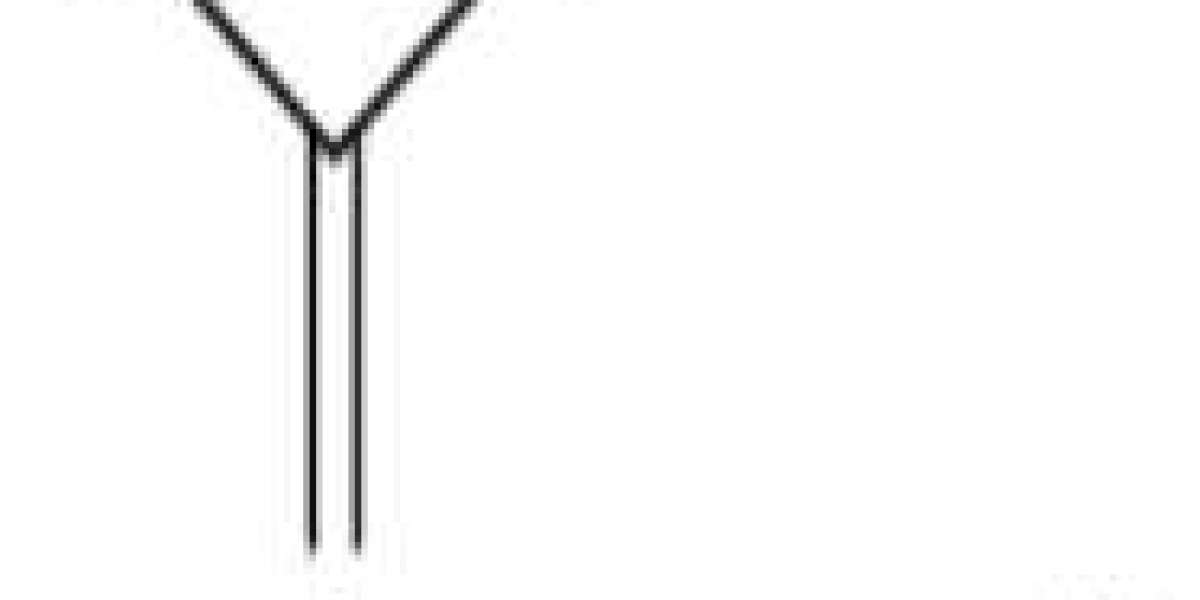
Ammonium bicarbonate is formed by combining carbon dioxide and ammonia:
CO2 + NH3 + H2O → (NH4)HCO3
Due to the thermal instability of ammonium bicarbonate, the reaction solution was kept cold, causing the product to precipitate as a white solid. About 100,000 tons were produced in this way in 1997. [3]
Ammonia gas is passed through a concentrated aqueous solution of sesquicarbonate (2:1:1 mixture of (NH4)HCO3, (NH4)2CO3 and H2O) to convert it to ammonium normal carbonate ((NH4)2CO3), which can Obtained in a crystalline state in solutions prepared at about 30 °C. Exposure of this compound to air releases ammonia which reverts to ammonium bicarbonate.
Compositions containing ammonium carbonate have long been known. They were once produced commercially and were formerly known as sal volatile or salt of hartshorn. It is obtained by dry distillation of nitrogen-containing organic matter such as hair, horn, and leather. In addition to ammonium bicarbonate, this material also contains ammonium carbamate (NH4CO2NH2) and ammonium carbonate ((NH4)2CO3). It is sometimes called ammonium bicarbonate. It has a strong ammonia odor, and after digestion with alcohol, the carbamate dissolves, leaving a residue of ammonium bicarbonate. [3]