Fasoracetam is a drug that has been claimed to enhance cognitive function and improve mood. While initial animal research has reported promising results, the science is in an early stage, and only limited data is available about its potential effects in human users. So what does the science currently say about this interesting drug? Read on to learn more about the possible uses, mechanisms, and side-effects of fasoracetam!
This post is not a recommendation or endorsement for the use of fasoracetam. The FDA has not approved this drug for any specific medical or other use, and the available research on it is still in a very early stage, without adequate data to come to any conclusions about its general efficacy or safety in humans. We have written this post for informational purposes only, and our goal is solely to inform people about what science currently says about fasoracetam’s mechanisms, potential effects, and possible side-effects.
What is Fasoracetam?
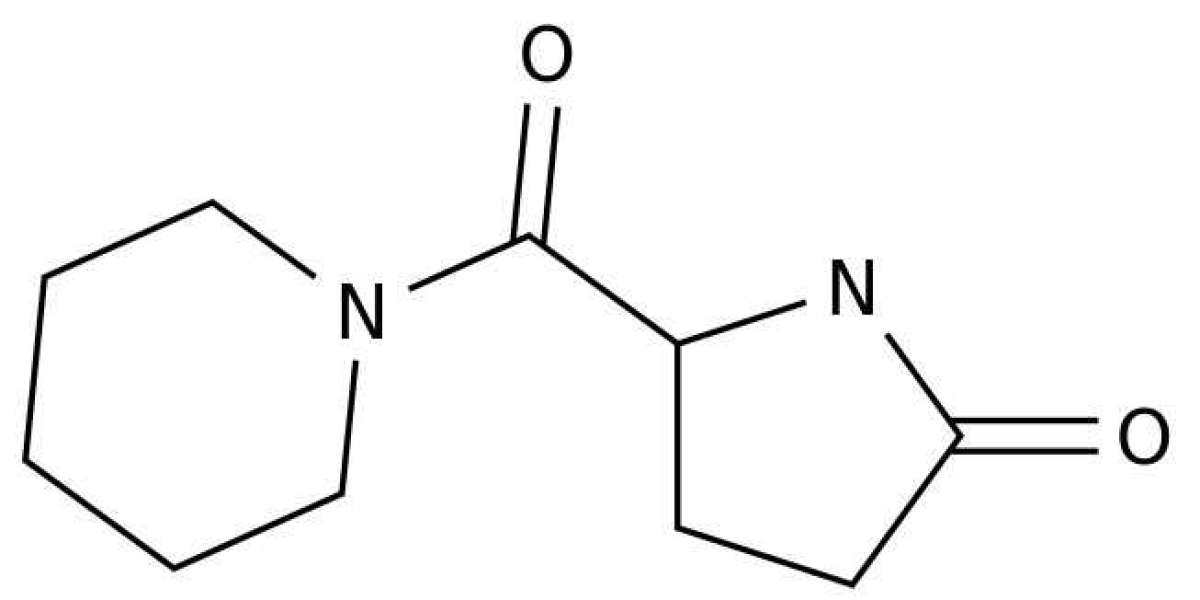
Fasoracetam – also sometimes known as NS-105, LAM-105, and NFC-1 – is a purported “nootropic” (a “cognitive enhancer” or “smart drug”) that belongs to the racetam family of drugs. It was first developed in the early 1990s by the Japanese pharmaceutical company Nippon Shinyaku, with the intention of treating vascular dementia. The company spent over $200 million developing fasoracetam: however, the drug failed to make it past phase 3 clinical trials due to a lack of efficacy, and was eventually abandoned.
In 2013, interest in fasoracetam was revived when a company called NeuroFix purchased the clinical data for the drug from Nippon Shinyaku. NeuroFix was later acquired by Aevi Genomic Medicine, which began clinical trials in 2016 in adolescents with ADHD, autism, or anxiety, who have mutations in the glutamate receptor gene. Fasoracetam is currently in phase 2 clinical trials.
However, this also means that fasoracetam has not yet been approved by the FDA for any specific medical use. Additionally, the fact that it is a relatively new substance means that it has not been extensively studied, and as such, it is not possible to make any firm conclusions about how effective or safe it may be – at least, not without a lot more research, including clinical trials in human users.
In this post, we will review some of the current evidence about fasoracetam’s mechanisms and purported effects. Nonetheless, as you read on, it’s important to keep in mind that all of this evidence is still in a very early stage, and any claims about this compound’s effects should, therefore, be taken with a healthy grain of salt!