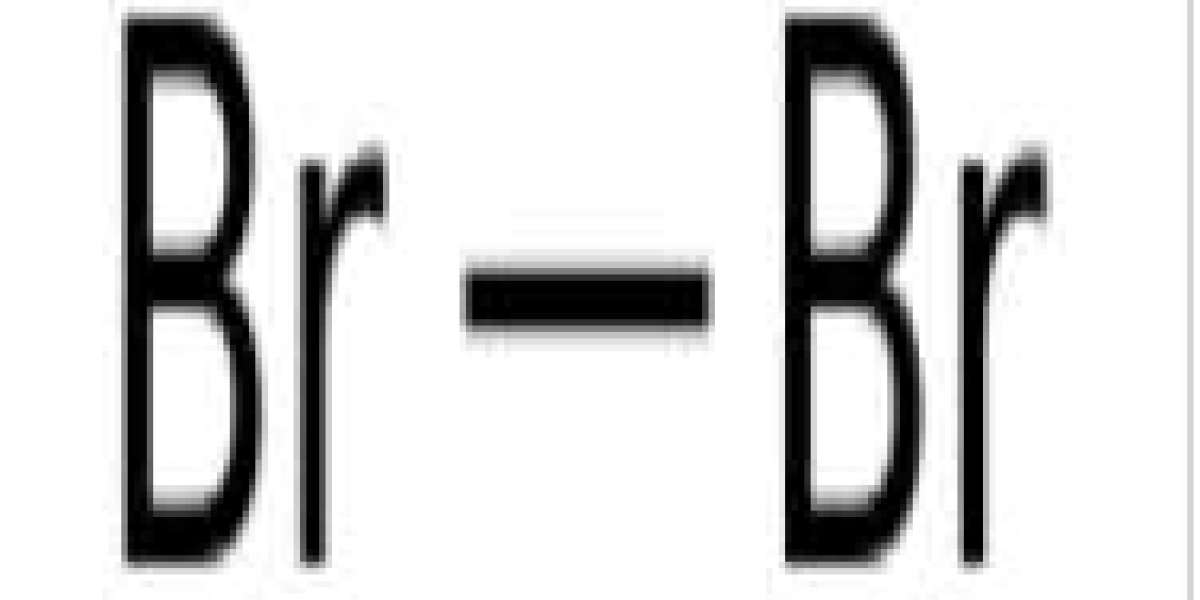A large amount of bromine salt reacts toxically with soluble bromide ions, causing bromine poisoning. However, a clear biological role for bromide ions and hypobromous acid has recently been elucidated, and bromine now appears to be an essential trace element for humans. The role of bioorganobromine compounds in marine organisms such as seaweed has long been known. As a drug, the simple bromide ion (Br−) has a depressant effect on the central nervous system, and bromide salts were once the main medical sedatives before being replaced by short-acting drugs. They retain a niche use as antiepileptic drugs.
Bromine was independently discovered by two chemists, Carl Jacob Löwig[6] and Antoine Balard[7][8] in 1825 and 1826 respectively. [9]
Löwig isolated bromine in 1825 from mineral water in his hometown of Bad Kreuznach. Löwig used a solution of mineral salts saturated with chlorine and extracted the bromine with ether. Evaporation of ether left a brown liquid. Using this liquid as a sample for his work, he applied for a position in the laboratory of Leopold Gmelin in Heidelberg. The publication of the results was delayed, and Ballard published his results first. [10]
Ballard found the bromine chemical in algae ash from the Montpellier Salt Flats. Seaweed is used to produce iodine, but also contains bromine. Ballard distilled bromine from a chlorine-saturated solution of seaweed ash. The properties of the resulting substance were intermediate between those of chlorine and iodine; he therefore tried to prove that the substance was iodine monochloride (ICl), but after failing, he was convinced that he had discovered a new element and named it muride, the source From Latin muria ("salt water").