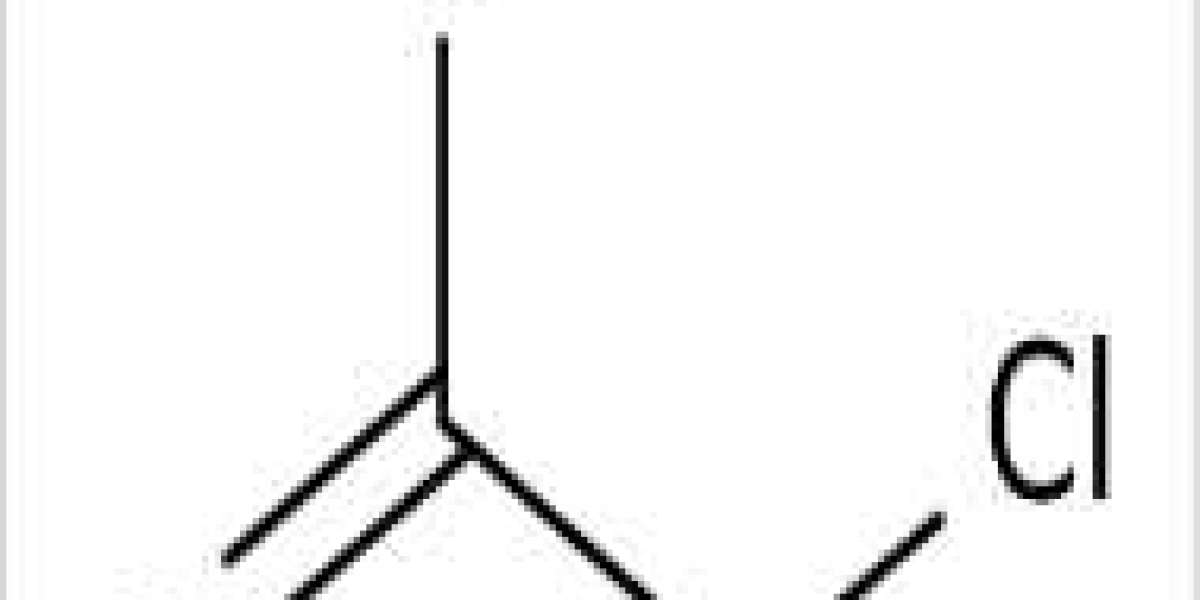
Chloroacetic acid, known industrially as monochloroacetic acid (MCA), is an organochlorine compound with the chemical formula ClCH2CO2H. Such carboxylic acids are useful building blocks in organic synthesis. It is a colorless solid. Related compounds are dichloroacetic acid and trichloroacetic acid.
Chloroacetic acid was first prepared (in impure form) in 1843 by the French chemist Félix LeBlanc (1813–1886) by chlorinating acetic acid in sunlight,[3] and the German chemist Reinhold Hoffmann Chloroacetic acid was prepared (in pure form) in 1857 (1831–1919) by refluxing glacial acetic acid in the presence of chlorine and sunlight,[4] then by the French chemist Charles Adolphe Wurtz by hydrolysis of chloroacetyl chloride (ClCH2COCl), also in 1857 Year.
Chloroacetic acid is produced industrially by two routes. The main method involves the chlorination of acetic acid, with acetic anhydride as a catalyst:
H3C−COOH + Cl2 → ClH2C−COOH + HCl
The disadvantage of this route is that dichloroacetic acid and trichloroacetic acid impurities are produced, which are difficult to separate by distillation:
H3C−COOH + 2 Cl2 → Cl2HC−COOH + 2 HClH3C−COOH + 3 Cl2 → Cl
3C−COOH + 3 HCl
The second method requires the hydrolysis of trichlorethylene:
ClHC=CCl2 + 2 H2O → ClH2C−COOH + 2 HCl
Hydrolysis is carried out in a solution of concentrated sulfuric acid (minimum 75%) at 130–140 °C. Unlike the halogenation route, this method yields high-purity products. However, the large amount of HCl released has led to the increasing popularity of the halogenated route. Approximately 420,000 tons are produced globally each year.
Most reactions take advantage of the high reactivity of the C-Cl bond.